
Antibody Drug Conjugates and Bioconjugation made in Bubendorf
An insight by Scott Miller, Ph.D. - Senior Scientific Advisor at CARBOGEN AMCIS AG

Since 2012 we have been actively building the Bioconjugation business in Bubendorf. In 2014, we have finished construction and qualification of the building 177 L113 & L114 cleanroom laboratories. Since then we have completed multiple projects, including the preparation of toxicology batches, analytical methods development, and stability studies for bioconjugates. These projects have included conjugation of a carbohydrate to an albumin for a targeted surgical application and a polysaccharide-protein conjugate as a potential synthetic vaccine.
CARBOGEN AMCIS uses site specific capabilities to advance projects
Another very complex project at CARBOGEN AMCIS has included manufacturing of a payload, with steps completed at CARBOGEN AMCIS in Manchester (UK) and at four of our sites in Switzerland. The final conjugation to prepare the ADC bulk drug substance was performed in our cleanroom laboratories in Bubendorf, with the final drug product Fill and Finish preparation completed by our colleagues in Riom (France). In addition, the analytical testing of the final drug product is partially performed by our group in Bubendorf, acting as an external analytical laboratory to Riom.
Continued growth for ADC and Bioconjugation therapeutic applications
While we have been establishing new Bioconjugation and ADC business, what has been happening in the industry? The growth of clinical trials has been trending steadily upwards. There have been new developments in antibody engineering and site-specific conjugations. In addition there continue to be new payloads and new therapeutic areas that are being studied.
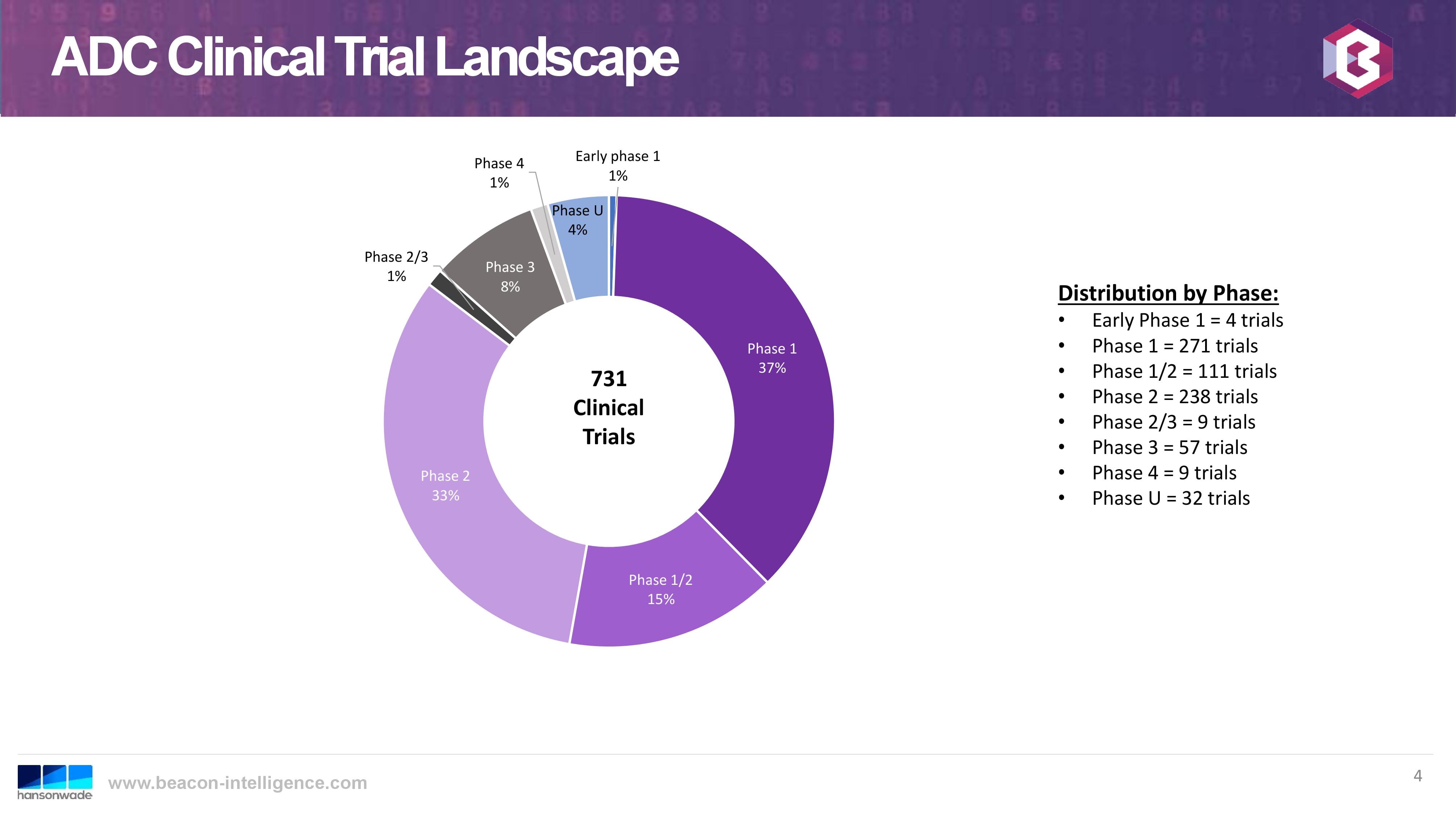
Recently, several members of the US sales team and the ADC group attended the World ADC Summit in San Diego. This year was the 10th anniversary of this annual event, providing an opportunity for presentations on the history and the current trends of this therapeutic approach. Overall, the outlook for continued growth in the therapeutic application of ADCs was very positive, even though there have also been some recent clinical disappointments. Letrishka Anthony of Beacon Intelligence, presented in her talk some interesting statistics. The total number of clinical trials has steadily increased, with a significant number in early phase:
Beacon Intelligence Hansonwade
This trend bodes well for CARBOGEN AMCIS, as so-far we have had the most success with customers entering the pre-clinical phase of development.The following slide from the same talk graphs the growth trend of clinical trials since 2014 (cut-off date for 2019 was August):

Beacon Intelligence Hansonwade
For ADCs the payloads being investigated continues to diversify. Note that our HiPo Development and Production teams have been involved in the manufacture of many of these payloads since 2005:

With an additional approval in 2019, the total number of approved ADCs on the market has risen to five, which I anticipate has further encouraged investment in this approach to the targeted treatment of diseases. The currently approved ADCs on the market are: Adcetris® (SeaGen), kadcyla® (Genentech), Besponsa® (Pfizer), Mylotarg® (Pfizer), and Polivy® (Genentech). While all approved ADCs are for oncology indications, there are also current clinical trials for potential applications as antibiotics, anti-HIV, and in the treatment of rheumatoid arthritis. The following slide, from the same World ADC presentation, shows a nice summary of approved ADCs:
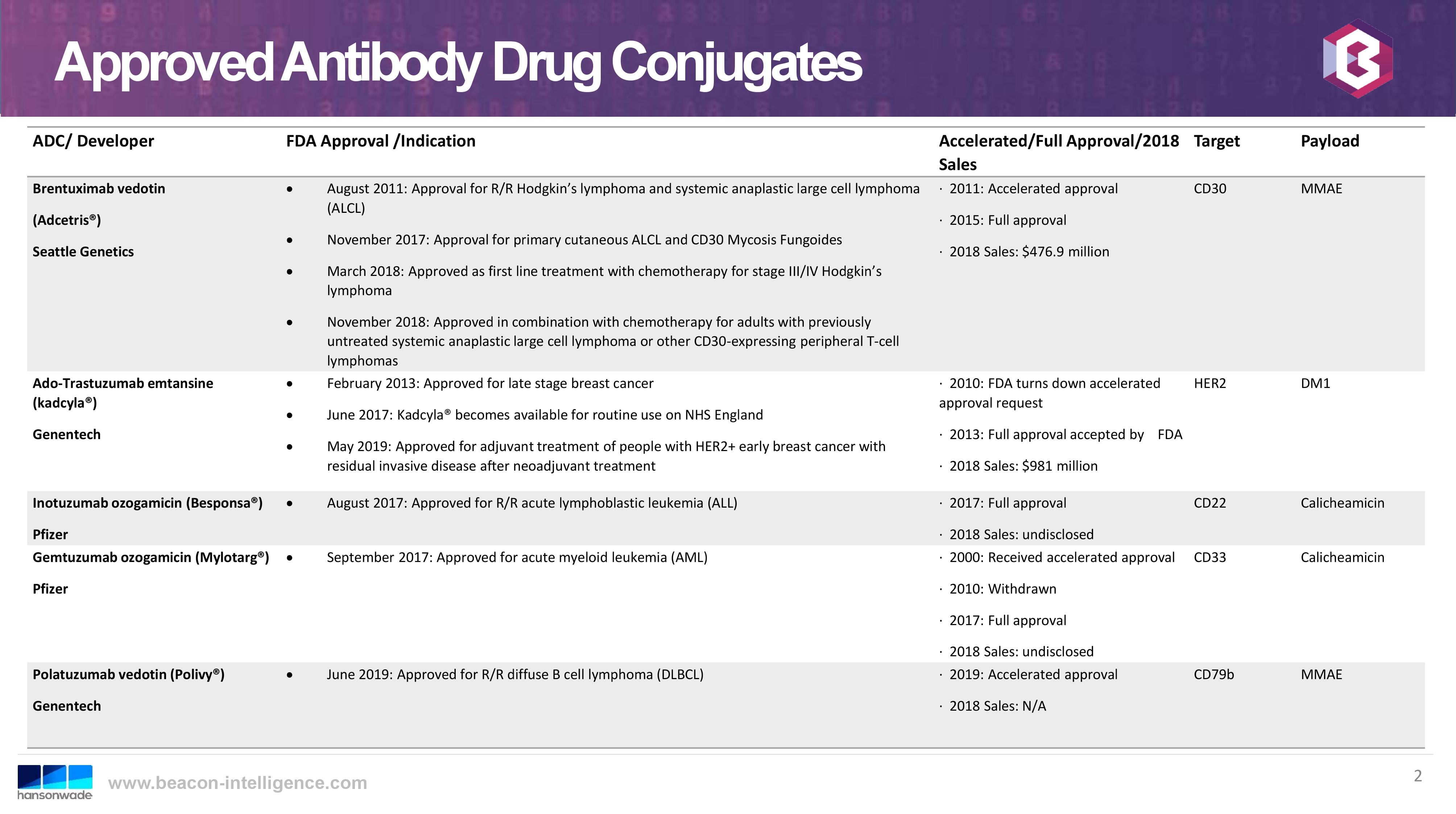
This ongoing industry growth is promising for the future growth of our ADC business. Along with our recent and current work supporting our customer’s ADC development we have also established capabilities in the bioconjugation and purification of non ADC bulk drug substances. This includes the demonstrated ability to develop and qualify a broad variety of analytical methods for diverse classes of bioconjugates and antibody drug conjugates.