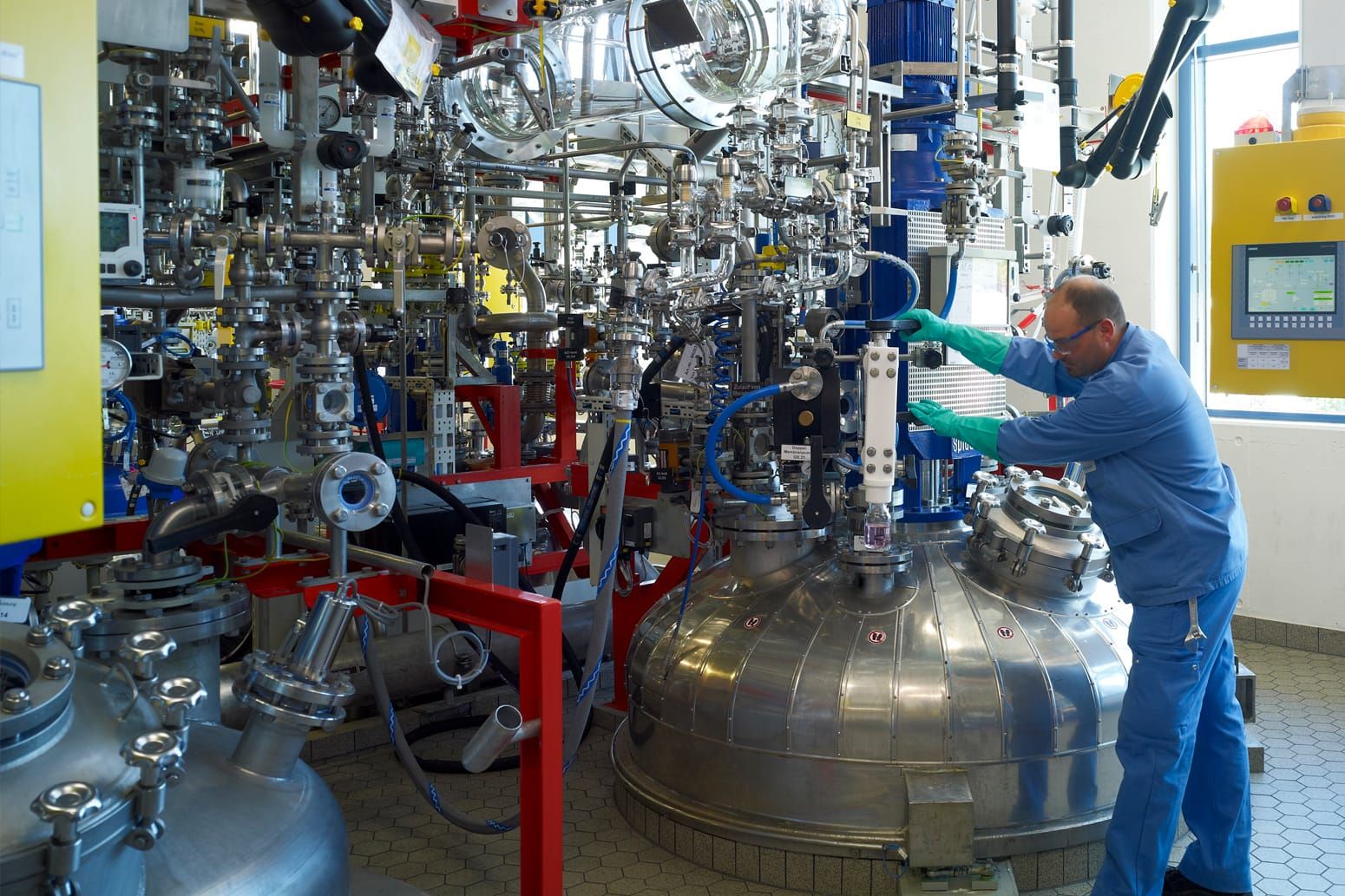





Our integrated services for Drug Substance (DS) and Drug Product (DP) provide innovative solutions to support timely and safe drug development. One stop shop project management from API to Drug Product with end-to-end services.
Drug Substance Drug Product
Fundamental to project success, we have a dedicated team of experienced Project Managers, operating within the organization. We work to an effective, tailored experienced project management process, focused on understanding and then addressing our customers' needs. With all project data being secure and accessible via SharePoint 24/7.
Flexible Project Management
Our state-of-the-art API and drug product facilities operate to the highest standards of current Good Manufacturing Practice (cGMP). Our fully integrated quality team (quality assurance and quality systems) conducts regular internal audits of each department within the company so we can maintain and continue to produce at the highest quality standards.
Quality Management
CARBOGEN AMCIS supports our customers through the often complex regulatory filing process and assists many clients in the preparation of the critical documentation phase. From Investigational New Drug Application (IND), through New Drug Application (NDA), to commercial, in-market supply of API, our dedicated staff in regulatory affairs has in excess of 50 years of cumulative experience.
Regulatory Affairs
Our service portfolio includes a wide range of fully integrated support functions that guide our customers through the life cycle of their drug substance and drug product, from development to commercial manufacturing. Our teams of experts manage your project along the full process to a commercial status.
Life Cycle Management
We provide advanced analytical support for drug substance and drug product development, offering robust method development, validation, and stability testing tailored to meet your requirements. Our state-of-the-art facilities ensure precision and excellence at every stage, guaranteeing product integrity and effectiveness over time.
Full Analytical Support
CARBOGEN AMCIS has state of the art facilities and controls to handle both drug substance and drug product of the highest potency and toxicity, under cGMP. Handling of molecules up to category 4+ such as Biologics, Peptides & ADCs.
Highly Potent Manufacturing
Our preparative chromatography capabilities have been developed and optimized over the last 20 years. Our experts are able to guide you through our extensive centralized solutions, with the goal to tailor an optimal preparative chromatography program; all based around your objectives, at every stage of the drug development cycle.
Chromatography Expertise
As pioneers in the highly potent landscape for over 14 years, CARBOGEN AMCIS has successfully delivered a significant number of drug-linker projects. Our first Antibody Drug Conjugates (ADC) project was delivered in 2005. Since then, our customers, ranging from small biotech to large pharmaceutical companies, have taken full advantage of our ADC and bioconjugation capabilities.
ADC & Bioconjugation
With profound expertise in the field of flow chemistry CARBOGEN AMCIS has the capability to help you controlling conditions and handling the risk as reactive volumes are small at any time. The range of applicable conditions such as temperature and pressure is becoming larger than in classical batch reactors. Not only will our experience in this method allow you a safer reaction and easier scale-up - it will also increase the purity of a product.
Flow ChemistryCARBOGEN AMCIS - Your Trusted CDMO Pharma Partner
As a Contract Development and Manufacturing Organization (CDMO), CARBOGEN AMCIS offers a complete service offering by being your steadfast partner, specializing in CDMO pharma services. Our expertise spans from Drug Substance to Drug Product development and manufacturing.
As a significant player in the pharmaceutical industry, our commitment is to offer a diverse range of CDMO services, tailored to meet the unique and evolving needs of our clients with the ultimate goal of treating patients.
What is CDMO?
CDMO stands for Contract Development and Manufacturing Organization. At CARBOGEN AMCIS, we’re not just an acronym; but a reliable partner to develop, manufacture and launch your drug on the market. With end-to-end solutions for drug development and manufacturing, we turn your ideas into reality.
Our CDMO Pharma Services
Step into our world of specialized CDMO pharma services, we are delivering high-quality solutions for your pharmaceutical needs. Imagine tailor-made solutions crafted with precision, covering the entire pharmaceutical development and manufacturing process. Discover more about an experienced CDMO pharma company in the industry. We provide services catering to the pharmaceutical sector, ensuring efficient and reliable development and manufacturing.
CDMO Drug Development and Manufacturing
Benefit from our extensive expertise in various aspects of drug development and manufacturing. Picture state-of-the-art manufacturing capabilities ensuring precision and quality in every product. Imagine having a partner with expertise in guiding your pharmaceutical product from concept to reality, all while maintaining a keen focus on innovation and efficiency. Fine-tune your processes with our specialized development services, optimizing efficiency and minimizing risks to bring your pharmaceutical vision to life.
CDMO Drug Substance
Highly potent compounds find their match at our state-of-the-art facilities, where we specialize in the development and manufacturing of drug substances that meet the highest industry standards.
CDMO Drug Product
From clinical to commercial stages, we excel in the formulation and production of liquid and lyophilized drug product forms.
Our expertise extends to handling highly potent compounds with precision and efficiency, ensuring the delivery of safe and effective pharmaceutical solutions.
Specialized CDMO Services
Explore our proficiency in specialized areas within the CDMO landscape.
CARBOGEN AMCIS is a CDMO specialized from drug substance to drug product development and manufaturing.
Experience our expertise in developing and manufacturing.
CARBOGEN AMCIS provides innovative solutions for drug development and supply to the pharmaceutical and bio-pharmaceutical industries that enable customers to bring new generation medicines to market. We have built up a portfolio of specialist services to give our customers the highest degree of flexibility possible: Highly Potent, Antibody Drug Conjugates & Bioconjugation, Chromatography, Crystallization, Stability Studies & Integrated Analytical Services, Flow Chemistry.
Beyond Pharmaceuticals - CDMO in Biotech and Life Sciences
We don’t just stop at pharmaceuticals; we extend our capabilities to cater to the dynamic fields of biotechnology and life sciences. Tailored solutions specifically crafted for the unique challenges posed by biopharmaceuticals. Experience our expertise in handling the complexities of biopharmaceutical development and manufacturing. See our commitment to advancing healthcare extend to the broader field of life sciences.


Your worldwide CDMO
CDMO with Global Footprint
Our sales force is spread across the US, Europe and Asia, and we serve customers on every continent, from pharmaceutical companies to biotech firms.
Pharma CDMO in Europe
Ready to make a global impact? Partner with a CDMO that not only leads globally but also sets industry standards and exceeds expectations. Discover the advantage of partnering with a global leader providing cutting-edge CDMO services in Europe, setting industry standards and exceeding expectations. Explore our extensive presence as a CDMO pharma company in Europe, offering not just services but unparalleled support and reliability.
Are you looking for an active pharmaceutical ingredient manufacturer in Europe? Or a drug product manufacturer in Europe? Find all our Europeans locations here.
Pharma CDMO in China
Embark on a journey of innovation and collaboration with our CDMO services in China. Our commitment to excellence extends globally, and our Shanghai site stands as a testament to our dedication. Discover more about our Shanghai site here. In the heart of one of the most dynamic pharmaceutical landscapes, we bring cutting-edge solutions, industry expertise, and a commitment to exceeding your expectations.
State-of-the-Art CDMO Facilities
Step into our CDMO facilities, a hub equipped to meet the highest industry standards. Here, quality and reliability are not just promises but integral parts of our services. Our facilities are designed to provide you with the assurance that your pharmaceutical products are in capable and secure hands.
Bubendorf
Vionnaz
Aarau
Neuland
St-Beauzire
Veenendaal
Shanghai
Manchester
When selecting CARBOGEN AMCIS as your CDMO partner, you are opting for a provider committed to delivering excellence, innovation, and reliability. If you’re prepared to turn your pharmaceutical vision into reality, feel free to reach out to us today.