
CDMO Europe: how to choose yours?
The main criteria to take into consideration
Choosing the right Contract Development and Manufacturing Organization (CDMO) for your pharmaceutical project in Europe is crucial for success
Here is a comprehensive guide to help you select the best CDMO:
1. Expertise and Capabilities
Seek out CDMOs with considerable experience in your specific field (for example biologics, small molecules, injectables or solid dose).
Ensure they can oversee your product from early development stages to large scale manufacturing; Verify their compliance with European regulations (such as EMA guidelines) and their track record of successful regulatory approvals. Evaluate their quality management systems and past regulatory audits outcomes—such as GMP certification.

2. Quality and Compliance
Ensure they have robust quality control and assurance procedures, including comprehensive testing and thorough documentation practices.
3. Capacity and Scalability
Determine if the CDMO can meet both your current and future production needs without delays or quality compromises. Ensure they can scale up operations as your product grows.
4. Technology and Innovation
Seek out those CDMOs investing in advanced technologies and innovative techniques to enhance production processes Prioritize those demonstrating a commitment to continuous improvement and staying current with industry trends.
5. Project Management and Communication
Evaluate the CDMO’s project management capabilities, focusing on their control over the timelines , milestone completion, and deliverables.
Ensure they maintain effective communication channels for regular updates and addressing any issues that arise.

6. Location and its Implications
Analyze the CDMO’s location for its impact on logistics and supply chain efficiency, as well as compliance with the local regulatory requirements.
7. Seek Feedback from Others
Look for customer testimonials and case studies to assess their past performance. Investigate their market reputation, any awards they have recieved or any issues they have encountered. Evaluate their pricing model for cost-effectiveness and transparency to avoid hidden fees.
Hidden fees are usually avoided if you look for transparency in costs.
8. Cultural Fit
Ensure their corporate culture and values align with yoursto guarantee a harmonious partnership.
In order to choose a CDMO that meets your requirements and ensures the success of your pharmaceutical project within Europe, consider these key factors.
European CDMO Facilities at CARBOGEN AMCIS
At CARBOGEN AMCIS, we have multiple facilities in Europe designed to meet a variety of needs.
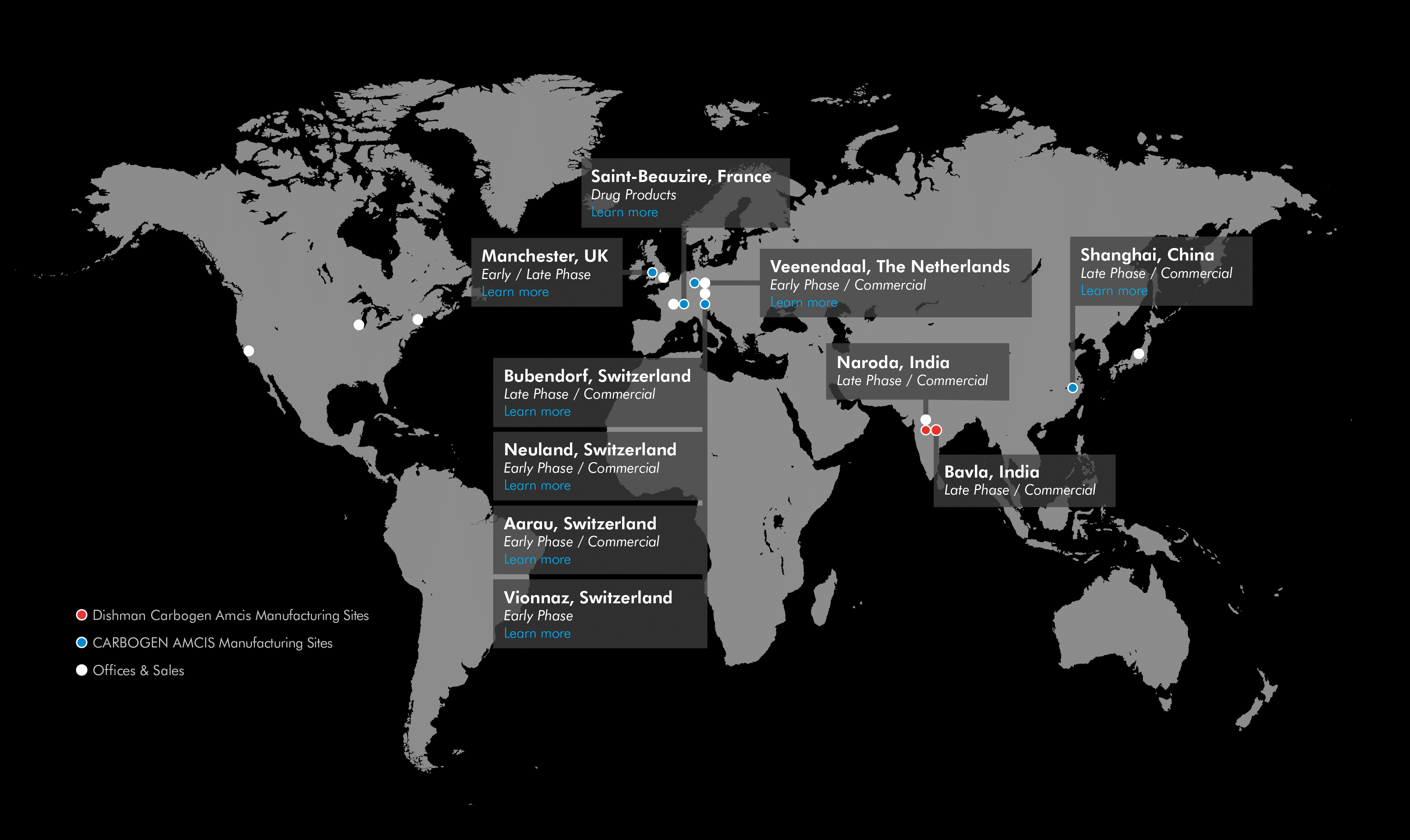
Four Swiss sites
The Bubendorf site is crucial for our company's active pharmaceutical ingredient (API) production, while the Aarau and Hunzenschwil (Neuland) sites are involved in various stages of pharmaceutical manufacturing and development. Our Vionnaz site focused on development HiPo and small scale production including chromatography and lyophilisation.
Our Swiss facilities have passed FDA and Swissmedic inspections, ensuring compliance with international standards.
Know more about our API / Drug Substance services
One French site
In France, we extended our experience in sterile drug product with a new state-of-the-art facility. Saint-Beauzire is focused specialises in the custom development and manufacturing of parenteral drug products, including complex formulations and highly potent compounds. The facility features two automated lines for liquid filling and lyophilization, as well as advanced developmental and analytical laboratories.
Know more about our Drug Product services
One English site
Our Manchester site suppors operations for our Swiss facilities but also operates as an independent entity, specializing in the manufacturing of cosmetic ingredients, intermediates, and fine chemicals.
One Dutch site
We also operate in Veenendaal, Netherlands. This site, situated in the heart of the Netherlands, is integral to the company's European operations. Originally established in 1946, the Veenendaal facility specializes in the production and development of high-quality cholesterol and lanolin derivatives, which are crucial for various pharmaceutical and cosmetic applications. We also provide Vitamin D and D2 analogs. The site provides comprehensive services for drug development and commercialization, ensuring high standards of quality and compliance with regulatory requirements.
Other sites
Outside Europe, CARBOGEN AMCIS also has a site in Shanghai.